Pfizer And PF-05221304 In NASH: The Lead Clinical Asset - Seeking Alpha
Investment Thesis
Pfizer (NYSE:PFE) is a large-cap ($232B) global biopharmaceutical company widely known for the development of the blockbuster cardiovascular drug, Lipitor. Lipitor is perhaps the most recognized statin drug that is used clinically and over the counter to lower bad cholesterol, LDL-C. Pfizer is also a proven pioneer of therapeutics for HIV infection, autoimmune, and gastrointestinal diseases as well as hematological and oncological ailments. Presently, it is clinically assessing the therapeutic effects of small drug molecules targeting the fibrotic liver disease, NASH.
Although a late-starter to clinically developing its NASH pipeline, Pfizer is now moving full speed with its NASH monotherapy and combination therapeutic clinical programs. There are three in-house drug candidates in clinical trials for NASH and/or NAFLD. Pfizer is developing lead asset, PF-05221304, an Acetyl-CoA carboxylase (ACC) inhibitor; PF-06835919, a Ketohexokinase (KHK) Inhibitor and PF-0686557; Diacylglycerol O-Acyltransferase 2 (DGAT2) Inhibitor.
This article focuses on the monotherapy trial of the lead NASH drug asset, PF-05221304, an ACC inhibitor, as a way to discerning the overall objective of Pfizer in NASH therapeutics development. Based on my analysis, I anticipate that PF-05221304 should induce clinically meaningful liver fat reduction, the primary clinical endpoint, in patients with NAFL or NASH. I elaborate in text.
PF-05221304 (ACC Inhibitor)
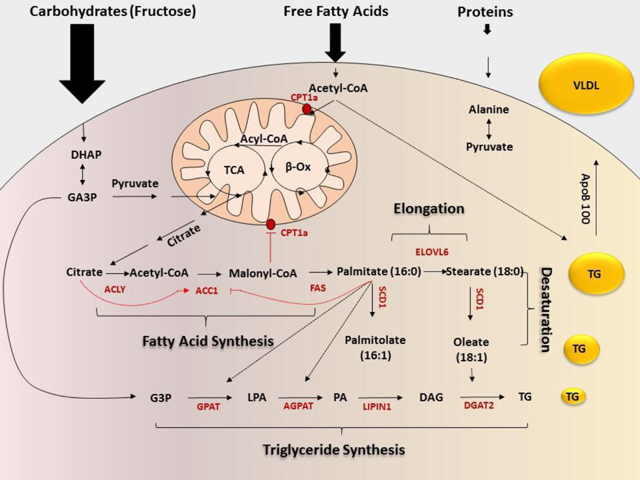
The liver parenchyma cells, hepatocytes, synthesize new fatty acids from excess carbohydrates, especially fructose by the process of de novo lipogenesis (DNL). For this reason, the primary substrates for DNL are carbohydrates, making DNL a major contributor to the free fatty acid flow into the liver. Although glucose feeds into DNL, it is regulated by the liver with excess glucose stored as glycogen.
Acetyl-CoA carboxylase (ACC) is an enzyme that exists as two isoforms. ACC1 is cytosolic and produces malonyl-CoA required for lipogenesis, while ACC2 is mitochondrial and is thought to be involved in the negative regulation of mitochondrial β-oxidation by modulating local malonyl-CoA levels. ACC catalyzes the irreversible carboxylation of acetyl-CoA to malonyl-CoA, which serves as a building block for fatty acid synthesis. Fig. 1 below depicts the process of hepatic DNL.
Fig. 1: Hepatic de novo lipogenesis
PF-05221304 is a potent, selective, and reversible dual inhibitor of ACC1/2, mechanistically designed to induce anti-steatosis effects by inhibiting the first step in DNL, the synthesis of fatty acids that potentially contributes to hepatic steatosis.
PF-05221304 is currently in Phase 2a trial in patients diagnosed with NASH or NAFLD with the 16 week top-line data readout expected in Q2/2019. Based on its mechanism of action, I expect to see a relative liver fat reduction of at least 30% from baseline in biopsied NASH patients after PF-05221304 treatment versus placebo. Notwithstanding, potential effects of PF-05221304 on NASH resolution and liver fibrosis will have to be specifically determined beyond the 16-week time-frame to assess its potential clinical value.
Actionable Event, Financials, And Risks
Pfizer has a clinical agreement with Novartis (NYSE:NVS) for combination trials involving their respective drug candidates. I am not really a fan of this alliance for the main reason that Tropifexor, an FXR agonist in clinical development by Novartis in Phase 2 NASH trial, may have the adverse events of dyslipidemia and pruritus associated with obeticholic acid by Intercept (NASDAQ:ICPT).
Pfizer may be a late starter to the NASH race, but I think PF-05221304 is a potential clinical winner as NASH therapeutics. Data readout in Q2/2019, if positive, should inform us on the future clinical plans for PF-05221304 in NASH, possibly Phase 2b trial. It is widely believed that Pfizer's growth will accelerate in 2019, and this is an opportune time to be a partaker of that growth!
While I believe that the likelihood of clinical success is high in my proposed thesis clinically meaningful hepatic fat reduction (primary outcome), all clinical trials are risky. Delays in patient recruitment, FDA hold, and negative data readout could all impact share the price.
Total revenues were $13.1B in Q1/2019 an increase of $211M versus Q1/2018.
Epilogue
Pfizer should have been at the forefront of NASH therapeutics development, given its history with blockbuster therapeutics for cardiovascular and metabolic disorders. Understandably, recognizing that they may not be tapping into the lucrative NASH addressable market anytime soon unless through acquisition, Dr. Morris Birnbaum, Snr. VP & CSO, stated
we are actively looking on the outside for opportunities to complement our internal program.
The full length article was discussed in more depth with members of my private investing community, Liver Therapy Forum
Members of my Liver Therapy Forum Marketplace service, receive:
-
My expertise as a PhD trained liver biomedical scientist to highlight drug candidates which are rarely similar but may have similar pharmacological target(s).
-
Exclusive access to full length in-depth research analytical articles and newsletters on liver therapeutics-focused investment opportunities.
-
Immediate/exclusive access to full length write-up from call interviews with CEOs/KOLs.
Visit my Landing Page to subscribe the low price of $32/month or $325 annually.
Disclosure: I/we have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
http://bit.ly/30WidDx

Comments
Post a Comment